Drug-Drug Interactions
서비스를 소개합니다
약물의 주요 대사는 간과 장에서 발생하며, 간 대사는 주로 Phase I enzyme (CYP)에 의해 일어나지만 Phase II enzyme인 UGT 등 비 CYP 효소를 통해서도 일어납니다. FDA 가이드라인에 따르면, 대사효소에 대해서 후보 약물이 CYP의 기질인지, 억제제인지, 유도제인지 여부를 평가해야 합니다. 약물 상호작용 (DDI)는 두 가지 이상의 약을 함께 복용할 경우, 하나의 약이 대사효소에 영향을 중 다른 약의 대사를 촉진하거나 억제할 수 있는 가능성을 분석합니다.
이런 서비스 유형을 제공합니다
서비스 유형 | CYP Phenotyping (7 assays, G230) | ||
목적 | 어떤 CYP 효소에 의해 약물이 대사되는지 확인 | ||
FDA 가이드라인 | l Enzyme Systems: Human liver subcellular fractions (e.g., microsomes, S9 and cytosol), Freshly isolated or cryopreserved human hepatocytes, Human recombinant enzymes. | ||
l Required Enzymes: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 및 CYP3A | |||
Assay Name | Item no. 1645 | Intrinsic clearance (CYP1A2) - US | |
Item no. 1646 | Intrinsic clearance (CYP2C9) - US | ||
Item no. 1647 | Intrinsic clearance (CYP2C19) - US | ||
Item no. 1648 | Intrinsic clearance (CYP2D6) - US | ||
Item no. 1649 | Intrinsic clearance (CYP3A4) - US | ||
Item no. 2384 | Intrinsic clearance (CYP2C8) - US | ||
Item no. 2387 | Intrinsic clearance (CYP2B6) - US | ||
실험농도 | 100 uM | ||
농도 개수 | 1개 농도 | ||
반복 실험 횟수 | Duplicate | ||
서비스 장소 | USA – St.Charles | ||
서비스 유형 | CYP Inhibition (8 assays, G232) | ||
목적 | 약물이 CYP 효소의 억제제로 작용하는지 평가 | ||
FDA 가이드라인 | l Enzyme Systems: Human liver microsomes, Human hepatocytes, Human recombinant enzymes. | ||
l Required Enzymes: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 및 CYP3A (CYP3A는 두 가지 기질 평가) | |||
Assay Name | Item no. 2064 | CYP1A inhibition (HLM, phenacetin substrate) | |
Item no. 2065 | CYP2B6 inhibition (HLM, bupropion substrate) | ||
Item no. 2244 | CYP2C8 inhibition (HLM, amodiaquine substrate) | ||
Item no. 2066 | CYP2C9 inhibition (HLM, diclofenac substrate) | ||
Item no. 1772 | CYP2C19 inhibition (HLM, omeprazole substrate) | ||
Item no. 1838 | CYP2D6 inhibition (HLM, dextromethorphan substrate) | ||
Item no. 1770 | CYP3A inhibition (HLM, midazolam substrate) | ||
Item no. 1769 | CYP3A inhibition (HLM, testosterone substrate) | ||
농도 개수 | 1개 농도 (10 uM) | ||
반복 실험 횟수 | Duplicate | ||
서비스 장소 | USA – St.Charles | ||
서비스 유형 | CYP Inhibition (8 assays) | ||
목적 | 약물이 CYP 효소의 억제제로 작용하는지 평가 | ||
FDA 가이드라인 | l Enzyme Systems: Human liver microsomes, Human hepatocytes, Human recombinant enzymes. | ||
l Required Enzymes: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 및 CYP3A (CYP3A는 두 가지 기질 평가) | |||
Assay Name | G232 | Item no. 2064 | CYP1A inhibition (HLM, phenacetin substrate) |
Item no. 2065 | CYP2B6 inhibition (HLM, bupropion substrate) | ||
Item no. 2244 | CYP2C8 inhibition (HLM, amodiaquine substrate) | ||
Item no. 2066 | CYP2C9 inhibition (HLM, diclofenac substrate) | ||
Item no. 1772 | CYP2C19 inhibition (HLM, omeprazole substrate) | ||
Item no. 1838 | CYP2D6 inhibition (HLM, dextromethorphan substrate) | ||
Item no. 1770 | CYP3A inhibition (HLM, midazolam substrate) | ||
Item no. 1769 | CYP3A inhibition (HLM, testosterone substrate) | ||
G361 | Item no. 4872 | CYP3A inhibition IC50 (HLM, testosterone substrate) - US | |
Item no. 4873 | CYP3A inhibition IC50 (HLM, midazolam substrate) - US | ||
Item no. 4874 | CYP2C19 inhibition IC50 (HLM, omeprazole substrate) - US | ||
Item no. 4875 | CYP2D6 inhibition IC50 (HLM, dextromethorphan substrate) - US | ||
Item no. 4876 | CYP1A inhibition IC50 (HLM, phenacetin substrate) - US | ||
Item no. 4877 | CYP2B6 inhibition IC50 (HLM, bupropion substrate) - US | ||
Item no. 4878 | CYP2C9 inhibition IC50 (HLM, diclofenac substrate) - US | ||
Item no. 4879 | CYP2C8 inhibition IC50 (HLM, amodiaquine substrate) - US | ||
농도 개수 | 1개 농도 (10 uM) 혹은 8개 농도 (100, 30, 10, 3, 1, 0.3, 0.1, 0.03 µM) | ||
반복 실험 횟수 | Duplicate | ||
서비스 장소 | USA – St.Charles | ||
서비스 유형 | Time-dependent CYP Inhibition (7 assays, +/- NADPH) | ||
목적 | 약물이 CYP 효소의 억제제로 작용하는지 평가 | ||
FDA 가이드라인 | l Enzyme Systems: Human liver microsomes, Human hepatocytes, Human recombinant enzymes. | ||
l Required Enzymes: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 및 CYP3A | |||
Assay Name | G234 | Item no. 2152 | Time-dependent CYP2D6 inhibition (HLM, dextromethorphan substrate + NADPH) - US |
G422 | Item no. 2755 | Time-dependent CYP2D6 inhibition (HLM, dextromethorphan substrate - NADPH) - US | |
Item no. 2184 | Time-dependent CYP3A inhibition (HLM, midazolam substrate + NADPH) - US | ||
Item no. 2185 | Time-dependent CYP3A inhibition (HLM, midazolam substrate - NADPH) - US | ||
Item no. 2544 | Time-dependent CYP2C9 inhibition (HLM, diclofenac substrate + NADPH) - US | ||
Item no. 2545 | Time-dependent CYP2C9 inhibition (HLM, diclofenac substrate - NADPH) - US | ||
Item no. 2546 | Time-dependent CYP2C19 inhibition (HLM, omeprazole substrate + NADPH) - US | ||
Item no. 2547 | Time-dependent CYP2C19 inhibition (HLM, omeprazole substrate - NADPH) - US | ||
Item no. 2634 | Time-dependent CYP1A inhibition (HLM, phenacetin substrate + NADPH) - US | ||
Item no. 2635 | Time-dependent CYP1A inhibition (HLM, phenacetin substrate - NADPH) - US | ||
Item no. 5215 | Time-dependent CYP2C8 Inhibition (HLM, amodiaquine substrate + NADPH) - US | ||
Item no. 5216 | Time-dependent CYP2C8 Inhibition (HLM, amodiaquine substrate - NADPH) - US | ||
Item no. 5302 | Time-dependent CYP2B6 Inhibition (HLM, bupropion substrate + NADPH) - US | ||
Item no. 5303 | Time-dependent CYP2B6 Inhibition (HLM, bupropion substrate - NADPH) - US | ||
Item no. 4873 | CYP3A inhibition IC50 (HLM, midazolam substrate) - US | ||
Item no. 4874 | CYP2C19 inhibition IC50 (HLM, omeprazole substrate) - US | ||
Item no. 4875 | CYP2D6 inhibition IC50 (HLM, dextromethorphan substrate) - US | ||
Item no. 4876 | CYP1A inhibition IC50 (HLM, phenacetin substrate) - US | ||
Item no. 4877 | CYP2B6 inhibition IC50 (HLM, bupropion substrate) - US | ||
농도 개수 | 1개 농도 (10 uM) 혹은 8개 농도 (100, 30, 10, 3, 1, 0.3, 0.1, 0.03 µM) | ||
반복 실험 횟수 | Duplicate | ||
서비스 장소 | USA – St.Charles | ||
서비스 유형 | CYP Induction (3 assays) | ||
목적 | 약물이 CYP 효소의 억제제로 작용하는지 평가 | ||
FDA 가이드라인 | l Enzyme Systems: Plateable, cryopreserved or freshly isolated, human hepatocytes (at least 3 donors), Immortalized hepatic cell lines | ||
l Required Enzymes: [Initially] CYP1A2, CYP2B6 and CYP3A4, [CYP3A4 induction 관찰되었을 경우] CYP2C8, CYP2C9, CYP2C19 추가 분석 필요 | |||
Assay Name | G384 | Item no. 2401 | CYP1A induction (human hepatocytes, enzyme activity, 3 donors) - US |
Item no. 2402 | CYP3A induction (human hepatocytes, enzyme activity, 3 donors) - US | ||
Item no. 2403 | CYP2B6 induction (human hepatocytes, enzyme activity, 3 donors) - US | ||
G345 | Item no. 3862 | CYP1A2 induction (human hepatocytes, mRNA level, 3 donors) - US | |
Item no. 3863 | CYP2B6 induction (human hepatocytes, mRNA level, 3 donors) - US | ||
Item no. 3865 | CYP3A4 induction (human hepatocytes, mRNA level, 3 donors) - US | ||
농도 개수 | 3개 농도 (1, 10, 100 uM) | ||
반복 실험 횟수 | Duplicate | ||
서비스 장소 | USA – St.Charles | ||
데이터 자료를 살펴보세요
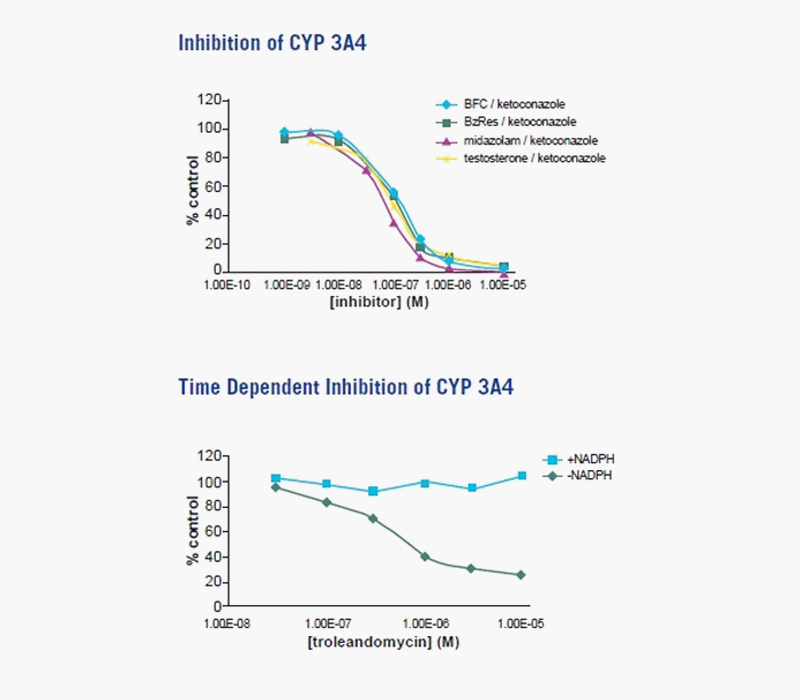
Figure 1. Reversible and Time-dependent CYP3A4 Inhibition. For reversible inhibition of CYP3A4, drug probe substrates or fluorometric substrates were incubated in the presence of increasing concentrations of a known CYP3A4 inhibitor, ketoconazole, in either recombinant CYP 3A4 (fluorometric substrates) or human liver microsomes (drug probe substrates). IC50s were generated by measuring percent inhibition of fluorescent products (fluorometric substrates) or formation of metabolites by HPLC-MS/MS (drug probes substrates). For time-dependent inhibition of CYP 3A4, IC50s were generated in the presence or absence of NADPH.
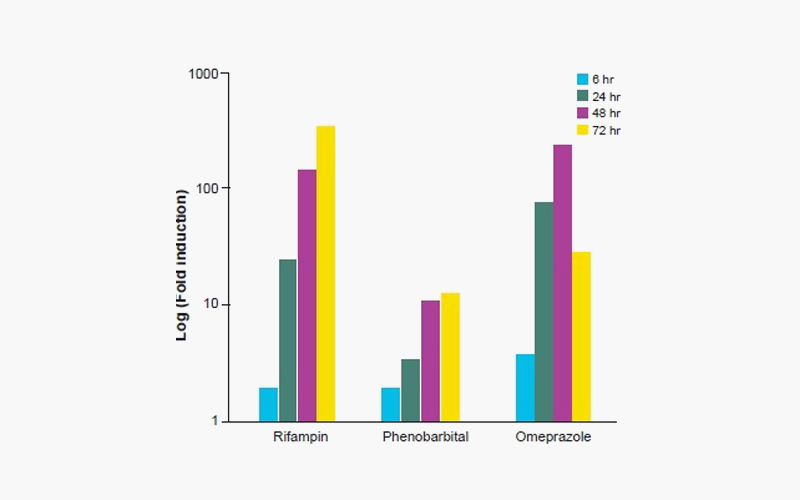
Figure 2. Time-dependent response of Refampin (10 uM) for CYP3A4, Phenobarbital (1 mM) for CYP2B6, and Omeprazole (100 uM) for CYP1A2
이런 분들에게 적극 추천합니다
규제기관 승인 제출을 위한 PK profile
바이오텍
규제기관 승인 제출을 위한 PK profile이 필요한 연구자에게 추천합니다
서비스는 이런 절차로 진행됩니다
-
유선 및 방문상담

서비스상담
실험항목 및 조건 논의 -
최초 견적서 전달

계약
실험의뢰서(CSF) 작성
(필요시) 계약서 작성 -
실험 물질 전달

시료 준비
(택배 혹은 방문수령) -
매주 FedEx 발송

시료 발송
Tracking no. 안내
Report due date 안내 -
실험종료 안내

결과제공
최종 보고서 전달
결과 검토 및 상담
결제 진행
