in vitro Toxicology
서비스를 소개합니다
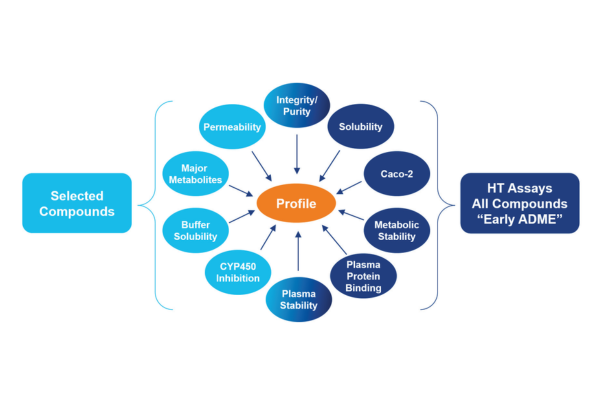
약물에 의해 발생하는 독성은 상용화 약물이 시장에서 철수되는 주요 원인입니다. 따라서 신약의 잠재적인 독성 여부는 초기 단계에서 검증되어야 하며, 후반 단계에서 발생하는 비용과 소요시간을 줄일 수 있는 방법입니다. Eurofins Discovery는 High resolution imaging 방법 및 다양한 세포 배양 방식과 수백 개의 인간 및 동물 세포주에서 독성 분석을 수행한 오랜 경험을 바탕으로 Cardiac Safety, Hepatotoxicity, Genotoxicity 등 약물에 의해 발생하는 잠재적인 독성을 광범위하게 분석하는 분석 서비스를 제공합니다.
이런 서비스 유형을 제공합니다
1. Hepatotoxicity |
|
|
Assay Name | Item no. | Panel no. |
Drug-Induced Liver Injury (DILI) Screening Panel - US | P385 | |
Cell Viability(HepG2, Galactose Medium, CellTiter-Glo) - US | 4801 | v |
Cell Viability(HepG2, Glucose Medium, CellTiter-Glo) - US | 4802 | v |
Cell Viability (HepG2, 72-hour, CellTiter-Glo) - US | 4803 | v |
Cytotoxicity (Human Primary Hepatocytes 24-hour) - US | P450 | |
Cell Viability (Human Primary Hepatocytes, 24-hour, CellTiter-Glo) - US | 5137 | v |
Cytotoxicity (Human Primary Hepatocytes 24-hour LDH-Glo) - US | 5879 | v |
Cytotoxicity (Human Primary Hepatocytes 48-hour) - US | P451 | |
Cell Viability (Human Primary Hepatocytes, 48-hour, CellTiter-Glo) - US | 5138 | v |
Cytotoxicity (human primary hepatocytes, 48-hour, LDH-Glo) - US | 5793 | v |
Cytotoxicity (HepG2; 5 endpoints; HCA) - US | G202 | |
Nuclear size - US | 1562 | v |
Mitochondrial membrane potential - US | 1563 | v |
Intracellular free calcium - US | 1564 | v |
Membrane permeability - US | 1565 | v |
Cell number (cytotox) - US | 1566 | v |
Hepatotoxicity (3D spheroids, human primary hepatocytes, 7-day, CellTiter-Glo) - US | 5787 | |
Hepatotoxicity IC50 (3D spheroids, human primary hepatocytes, 7-day, CellTiter-Glo) - US | 5786 | |
Cell Viability (Rat Primary Hepatocytes, 48-hour, CellTiter-Glo) - US | 5851 | |
Cell Viability (HepG2, Galactose Medium) - US | 4621 | |
Cell Viability (HepG2, Glucose Medium) - US | 4622 | |
Cell Viability (Mouse Primary Hepatocytes, 48-hour, CellTiter-Glo) - US | 5106 | |
Cell Viability (Rat Primary Hepatocytes, 24-hour, CellTiter-Glo) - US | 5107 | |
Cell Viability (HepG2, 24-hour, CellTiter-Glo) - US | 5135 | |
Cell Viability (HepG2, 48-hour, CellTiter-Glo) - US | 5136 | |
Cytotoxicity (HepG2 24-hour LDH-Glo) - US | 5880 | |
Cytotoxicity (HepG2 48-hour LDH-Glo) - US | 5881 | |
Cellular Stress (HRPTEpiC; Cell Viability; Cellular Stress; Kidney Injury) - US | 970030 | |
Cellular Stress (HPH; Cell Viability; HSP27; Apoptosis) - US | 970010 | |
Cytotoxicity (HPH; Cell Viability; ROS; MMP) - US | 970011 | |
Cytotoxicity (HPH; Cell Viability; Apoptosis) - US | 970012 |
|
2. Genotoxicity
|
|
|
Assay Name | Item no. | Panel no. |
Ames fluctuation test - US | G175 | |
Ames fluctuation test (TA1535 - S9) - US | 3011 | v |
Ames fluctuation test (TA1535 + S9) - US | 3012 | v |
Ames fluctuation test (TA1537-S9) - US | 3582 | v |
Ames fluctuation test (TA1537 + S9) - US | 3583 | v |
Ames fluctuation test (TA98 - S9) - US | 432 | v |
Ames fluctuation test (TA100 - S9) - US | 520 | v |
Ames fluctuation test (TA98 + S9) - US | 521 | v |
Ames fluctuation test (TA100 + S9) - US | 522 | v |
Micronucleus Panel - US | G344 | |
Micronucleus (CHO - S9, HCA) - US | 1560 | v |
Micronucleus (CHO + S9, HCA) - US | 1684 | v |
3. Cytotoxicity |
| |
Assay Name | Item no. | |
Cytotoxicity (non-standard) - US | 1537 | |
Cytotoxicity (HUMSC; Cell Viability; Apoptosis; Cell Cycle) - US | 970041 | |
Cytotoxicity (HUVEC; Cell Viability; Apoptosis; Cell Cycle) - US | 970520 | |
Cytotoxicity (NHDF-neo; Cell Viability; Apoptosis; Cell Cycle) - US | 970021 | |
Cellular Stress (HUMSC; Cell Viability; HSP2) - US | 970050 | |
Cellular Stress (HUVEC; Cell Viability; HSP27) - US | 970040 | |
Cellular Stress (NHDF-neo; Cell Viability; HSP27) - US | 970020 | |
Cell viability (HUMSC) - US | 4574 | |
Cell viability (HUVEC) - US | 4575 | |
Cell Viability (NHDF-neo, 24-hour, CellTiter-Glo) - US | 4576 |
데이터 자료를 살펴보세요
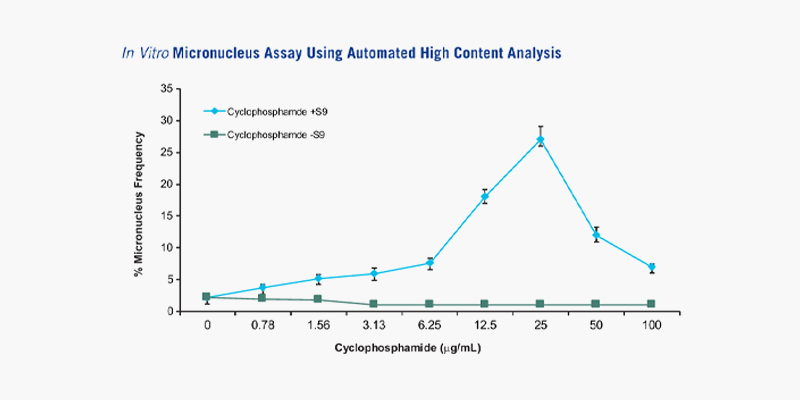
[Micronuclei frequency with cyclophosphamide treatment: Cyclophosphamide, an indirect clastogen, induces an increase of micronuclei frequency only in the presence of metabolic activation (+S9 fraction)]

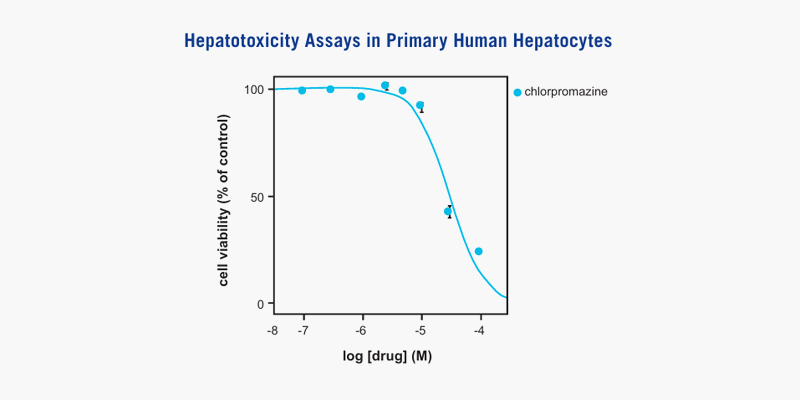
[Dose dependent effects of chlorpromazine on cell viability in sandwich cultured cryopreserved human primary hepatocytes.]
서비스는 이런 절차로 진행됩니다
-
유선 및 방문상담

서비스상담
실험항목 및 조건 논의 -
최초 견적서 전달

계약
실험의뢰서(CSF) 작성
(필요시) 계약서 작성 -
실험 물질 전달

시료 준비
(택배 혹은 방문수령) -
매주 FedEx 발송

시료 발송
Tracking no. 안내
Report due date 안내 -
실험종료 안내

결과제공
최종 보고서 전달
결과 검토 및 상담
결제 진행