Neuron Panels

생명유지 필수기관인 중추 신경계 (CNS, Central Nerve System)와 연관된 다양한 target들로 구성된 panel 이며, 이를 통해 개발중인 약물의 MOA 및 off-target effect를 확인할 수 있습니다.
문의하기서비스를 소개합니다
심혈관계 부작용 (CV risk)와 더불어 약물 부작용으로서 높은 비율로 발생하는 것이 신경독성 (Neurotoxicity) 입니다. 이는 정서장애, 통증, 인지 장애와 같은 질환이 동반 질환으로 나타나기 때문에 신경독성 부작용은 약물의 시장 경쟁력을 떨어지는 주요 원인으로 알려져 있습니다. 따라서 약물개발의 초기 단계부터 관련 신경독성에 대한 안전성을 확인해야 신약 개발의 성공 가능성일 높일 수 있습니다.
Eurofins Discovery는 CNS 관련 타겟 뿐만 아니라 통증 (Pain), 발작 (Seizure)을 일으키는 기전에 관련된 타겟으로 구성된 다양한 Panel 분석 서비스를 제공합니다. 특히 CNS 치료 약물의 경우, FDA 지침에 따른 약물 오남용 가능성 평가가 반드시 선행되어야 하며, 이 과정에 활용할 수 있는 Drug Abuse Panel을 별도로 구성하여 제공합니다.
![]() CNS Target Panel / SafetyScreen CNS Panel
CNS Target Panel / SafetyScreen CNS Panel
중추 신경계(CNS, Central Nerve System)와 연관된 다양한 target들로 구성된 panel입니다.
2종류의 Panel(CNS Target-대만/SafetyScreen CNS-프랑스)이 있으며, SafetyScreen CNS에서 5개의 Assay(연두색 표시)가 더 포함되어 있습니다.
Enzyme | GPCR | Ion Channel | |
ATPase (Na+/K+), Brain, Pig | Adenosine A1 | Dopamine D3 | Calcium Channel L-Type, Benzothiazepine |
Carbonic Anhydrase II | Adenosine A2A | Dopamine D4.4 | Calcium Channel L-Type, Dihydropyridine |
Catechol-O-Methyl Transferase (COMT) | Adenosine A2B | Endothelin ETA | Calcium Channel L-Type, Phenylalkylamine |
Cholinesterase, Acetyl, ACES | Adenosine A3 | Glutamate, Metabotropic, mGlu5 | Calcium Channel N-Type |
CYP450, 1A2 | Adrenergic alpha1A | Melatonin MT1 | GABAA, Chloride Channel, TBOB |
CYP450, 2C19 | Adrenergic alpha1B | Muscarinic M1 | GABAA, Flunitrazepam, Central |
CYP450, 2D6 | Adrenergic alpha2A | Muscarinic M2 | GABAA (alpha1/beta2/gamma2) |
CYP450, 3A4 | Adrenergic alpha2B | Muscarinic M3 | Glutamate, AMPA |
GABA Transaminase | Adrenergic alpha2C | Muscarinic M4 | Glutamate, Kainate |
Monoamine Oxidase MAO-A | Adrenergic beta1 | Muscarinic M5 | Glutamate, NMDA, Agonism |
Monoamine Oxidase MAO-B | Adrenergic beta2 | Tachykinin NK1 | Glutamate, NMDA, Phencyclidine |
Peptidase, Angiotensin Converting Enzyme | Bradykinin B2 | Neuropeptide Y Y1 | Glutamate, Glycine, Strychnine-intensive |
Peptidase, CTSB (Cathepsin B) | Calcitonin Gene-Related Peptide CGRP1 | Neuropeptide Y Y2 | Glycine, Strychnine-Sensitive |
Peptidase, CTSL (Cathepsin L) | Cannabinoid CB1 | Opiate delta1 (OP1, DOP) | Nicotinic Acetylcholine alpha4beta2, Cytisine |
Phosphodiesterase PDE4D2 | Cannabinoid CB2 | Opiate kappa (OP2, KOP) | Nicotinic Acetylcholine alpha7, Bungarotoxin |
Protein Serine/Threonine Kinase, MAPK14 (p38alpha) | Cholecystokinin CCK1 (CCKA) | Opiate mu (OP3, MOP) [3H]morphine | Potassium Channel [SKCA] |
Protein Tyrosine Kinase, EGF Receptor | Cholecystokinin CCK2 (CCKB) | 5-HT4E Human Serotonin | Purinergic P2X |
Protein Tyrosine Kinase, Insulin Receptor | Dopamine D1 | Orexin OX2 | Serotonin (5-Hydroxytryptamine) 5-HT3 |
Tyrosine Hydroxylase | Dopamine D2L | Orexin OX1 | Sodium Channel, Site 2 |
JNK1 Human CMGC Kinase Enzymatic LANCE Assay [Km ATP] | Dopamine D3 | Oxytocin | Nuclear Hormone Receptor |
Transporter | Dopamine D4.4 | Serotonin (5-Hydroxytryptamine) 5-HT1A | Androgen (Testosterone) |
Transporter, Adenosine | Endothelin ETA | Serotonin (5-Hydroxytryptamine) 5-HT1B | Estrogen ERalpha |
Transporter, Norepinephrine (NET) | GABAB1B | Serotonin (5-Hydroxytryptamine) 5-HT2A | Glucocorticoid |
Transporter, Dopamine (DAT) | Glutamate, Metabotropic, mGlu5 | Serotonin (5-Hydroxytryptamine) 5-HT2B | Others |
Transporter, GABA | Histamine H1 | Serotonin (5-Hydroxytryptamine) 5-HT2C | BZDp (TSPO) |
Transporter, Serotonin (5-Hydroxytryptamine) (SERT) | Histamine H3 | Serotonin (5-Hydroxytryptamine) 5-HT7 | TNF-alpha |
Vasopressin V1A | Sigma, Non-Selective | ||
![]() Drug Abuse Safety Panel/Drug Abuse Potential Panel
Drug Abuse Safety Panel/Drug Abuse Potential Panel
Drug Abuse(약물 남용)에 관련된 2 종류의 Panel 서비스(Drug Abuse Safety Panel-대만/Drug Abuse Potential Panel-프랑스)를 제공합니다.
2017, FDA guideline, Assessment of Abuse Potential of Drugs에 따른 Assay로 구성되어 있습니다.
Drug Abuse Safety-48 assay | Drug Abuse Potential-44 assay | ||
Enzyme | |||
104010 | Cholinesterase, Acetyl, ACES | ||
195010 | Tyrosine Hydroxylase | ||
GPCR | |||
203110 | Adrenergic alpha1A | 2338 | Adrenergic alpha1A |
203210 | Adrenergic alpha1B | ||
203400 | Adrenergic alpha1D | ||
203630 | Adrenergic alpha2A | 13 | Adrenergic alpha2A |
203710 | Adrenergic alpha2B | 1344 | Adrenergic alpha2B |
203810 | Adrenergic alpha2C | 16 | Adrenergic alpha2C |
204010 | Adrenergic beta1 | 18 | Adrenergic beta1 |
204110 | Adrenergic beta2 | 20 | Adrenergic beta2 |
217050 | Cannabinoid CB1 | 4708 | Cannabinoid CB1 |
217100 | Cannabinoid CB2 | 37 | Cannabinoid CB2 |
219500 | Dopamine D1 | 44 | Dopamine D1 |
219700 | Dopamine D2S | 1322 | Dopamine D2S |
228610 | GABAB1A | ||
228710 | GABAB1B | ||
236400 | Glutamate, Metabotropic, mGlu2 | ||
252610 | Muscarinic M1 | 91 | Muscarinic M1 |
252710 | Muscarinic M2 | 93 | Muscarinic M2 |
252810 | Muscarinic M3 | 95 | Muscarinic M3 |
252910 | Muscarinic M4 | 96 | Muscarinic M4 |
253010 | Muscarinic M5 | 97 | Muscarinic M5 |
260130 | Opiate delta1 (OP1, DOP) | 114 | Opiate delta1 (OP1, DOP) |
260210 | Opiate kappa (OP2, KOP) | 4461 | Opiate kappa (OP2, KOP) |
260430 | Opiate mu (OP3, MOP) [3H]morphine | 118 | Opiate mu (OP3, MOP) [3H]morphine |
299030 | Orexin OX1 | 1987 | Orexin OX1 |
299002 | Orexin OX2 | 1988 | Orexin OX2 |
271110 | Serotonin (5-Hydroxytryptamine) 5-HT1A | 131 | Serotonin (5-Hydroxytryptamine) 5-HT1A |
271230 | Serotonin (5-Hydroxytryptamine) 5-HT1B | ||
271650 | Serotonin (5-Hydroxytryptamine) 5-HT2A | 471 | Serotonin (5-Hydroxytryptamine) 5-HT2A |
271700 | Serotonin (5-Hydroxytryptamine) 5-HT2B | 1333 | Serotonin (5-Hydroxytryptamine) 5-HT2B |
271800 | Serotonin (5-Hydroxytryptamine) 5-HT2C | 1003 | Serotonin (5-Hydroxytryptamine) 5-HT2C |
Drug Abuse Safety-48 assay | Drug Abuse Potential-44 assay | ||
Ion Channel | |||
216000 | Calcium Channel N-Type | 164 | Calcium Channel N-Type |
226600 | GABAA, Flunitrazepam, Central | 28 | GABAA, Flunitrazepam, Central |
232600 | Glutamate, AMPA | 64 | Glutamate, AMPA |
232710 | Glutamate, Kainate | 65 | 65 |
232810 | Glutamate, NMDA, Agonism | 66 | Glutamate, NMDA, Agonism |
233000 | Glutamate, NMDA, Phencyclidine | 124 | Glutamate, NMDA, Phencyclidine |
299032 | Nicotinic Acetylcholine alpha7, Bungarotoxin | 3010 | Nicotinic Acetylcholine alpha7, Bungarotoxin |
258660 | Nicotinic Acetylcholine alpha7, Methyllycaconitine | 170 | GABAA, TBPS |
271910 | Serotonin (5-Hydroxytryptamine) 5-HT3 | 411 | Serotonin (5-Hydroxytryptamine) 5-HT3 |
169 | Sodium Ion Channel, Batrachotoxinin(Site 2) | ||
3029 | nAChR (alpha4/beta2) | ||
3051 | GABAA (alpha1/beta2/gamma2) | ||
68 | Glutamate, Glycine (Strychnine-Insensitive) | ||
Nuclear Hormone Receptor | |||
226010 | Estrogen ERalpha | 933 | Androgen |
226050 | Estrogen ERbeta | 152 | Estrogen ERbeta |
232030 | Glucocorticoid | 469 | Glucocorticoid |
Transporter | |||
220320 | Transporter, Dopamine (DAT) | 52 | Transporter, Dopamine (DAT) |
226400 | Transporter, GABA | 60 | Transporter, GABA |
204410 | Transporter, Norepinephrine (NET) | 355 | Transporter, Norepinephrine (NET) |
274030 | Transporter, Serotonin (5-Hydroxytryptamine) (SERT) | 439 | Transporter, Serotonin (5-Hydroxytryptamine) (SERT) |
![]() Neurodegeneration Phenotypic Panel
Neurodegeneration Phenotypic Panel
Cell based Phenotypic Panel로서, 신경 퇴행성 질환 후보 약물 처리 시, ≥ 50% increase of neurite outgrowth를 통해 효능을 확인할 수 있는 서비스입니다.
FAMILY | NAME | ITEM NUMBER | PROTEIN NAME | ORGANISM | ASSAY SUB TYPE |
Cell Based Phenotypic | Alpha-Synuclein A53T Parkinson’s Disease Genetic Cell Based Agonist Neurite Outgrowth Assay, Panlabs | 332400-0 | SYUA_HUMAN | Human | Cell Based |
Cell Based Phenotypic | APOEε4 C130R Sporadic Alzheimer’s Disease Genetic Cell Based Agonist Neurite Outgrowth Assay, Panlabs | 332440-0 | APOE_HUMAN | Human | Cell Based |
Cell Based Phenotypic | APP- PS1- MAPT Familial Alzheimer’s Disease Genetic Cell Based Agonist Neurite Outgrowth Assay, Panlabs | 332460-0 | A4_HUMAN | Human | Cell Based |
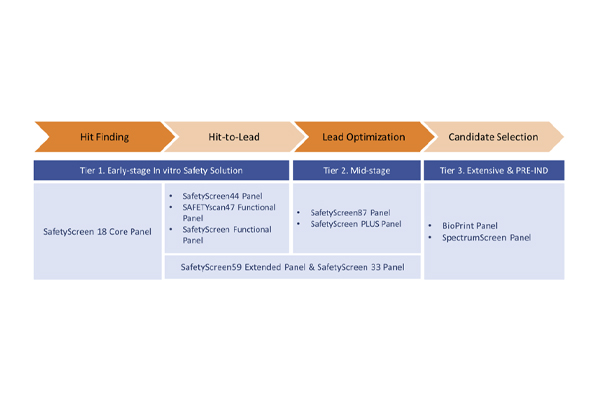
이런 서비스 유형을 제공합니다
서비스 유형 | CNS Target Panel | SafetyScreen CNS panel | Drug Abuse Safety Panel | Drug Abuse Potential Panel |
Item No. | PP280 | P411 | PP279 | P293 |
Assay 수 | 94 | 99 | 48 | 44 |
Sample Volume | 150 μl of 10 mM stock | 230 μl of 10 mM stock | 150 μl of 10 mM stock | 105 μl of 10 mM stock |
반복횟수 | Duplicate | Duplicate | Duplicate | Duplicate |
TAT | 10 Days | 15 Days | 10 Days | 15 Days |
서비스 장소 | Panlabs, Taiwan | Cerep, France | Panlabs, Taiwan | Cerep, France |
서비스 유형 | Pain Target Panel* | Alzheimer’s MoA Panel | Parkinson’s MoA Panel | Neurodegeneration Phenotypic Panel |
Item No. | PP293 | PP284 | PP285 | PP283 |
Assay 수 | 44 | 41 | 27 | 3 |
Sample Volume | 1 농도:150 μl of 10 mM stock | 120 μl of 10 mM stock | 120 μl of 10 mM stock | 240 μl of 10 mM stock |
반복횟수 | Duplicate | Duplicate | Duplicate | 8 concentration, Duplicate |
TAT | 10 Days | 10 Days | 10 Days | 30 Days |
서비스 장소 | Panlabs, Taiwan | Panlabs, Taiwan | Panlabs, Taiwan | Panlabs, Taiwan |
서비스는 이런 절차로 진행됩니다
-
유선 및 방문상담

서비스상담
실험항목 및 조건 논의 -
최초 견적서 전달

계약
실험의뢰서(CSF) 작성
(필요시) 계약서 작성 -
실험 물질 전달

시료 준비
(택배 혹은 방문수령) -
매주 FedEx 발송

시료 발송
Tracking no. 안내
Report due date 안내 -
실험종료 안내

결과제공
최종 보고서 전달
결과 검토 및 상담
결제 진행