Oncology in vivo models
서비스를 소개합니다
Eurofins Discovery의 in vivo oncology model은 CDX 및 PDX model 모두 보유하고 있으며, 약물 투여 후 flow cytometry, RT-PCR, ELISA, Western blot, histopathology, bioanalysis 등 ex vivo analysis를 통해 다양한 조직에 대한 약물의 항암 효과를 평가할 수 있습니다.
l Small molecule & Large molecule 모두 사용 가능
l Readout 측정을 위한 다양한 방법 선택 가능
- Conventional
caliper tumor measurements
- Conventional
macroscopic metastatic nodules
- In vivo
imaging with IVIS® Spectrum
- Survival
analysis with humane endpoints
- Flow
cytometry analysis with leukemia/lymphoma progression
- Histopathology,
cytokine, flow cytometry, RT-qPCR, ELISA, Western blot
- Pharmacokinetics (PK) bioanalysis
l 약물 투여방법 (PO, SC, IP, IV, Itu injection) 및 주기 선택 가능:
l
Custom Model
Development: 고객이 제공하는 세포 혹은
Eurofins Discovery가 보유한 OncoPanel service에 사용되는 300개 이상의 cancer cell line을 사용해 새로운 in vivo model 구축 가능
이런 서비스 유형을 제공합니다
Validated
Syngeneic Models
Mouse cancer cell line을 피하 이식 (Subcutaneous) 또는 조직 이식 (Orthotopic) 을 통해 생쥐에 주입하며, Chemotherapy 및 immunotherapy에 대해 검증된 모델을 제공합니다.
Model Name | Item no. | Cell Lines Used in Mouse Model | |
Syngeneic, Breast, 4T1 | 578590 | ATCC | CRL-2539 |
Syngeneic, Colon, CT26.WT | 578600 | ATCC | CRL-2638 |
Syngeneic, Colon, MC-38 | 578620 | Keradast | ENH204-FP |
Syngeneic, Kidney, Renca | 578630 | ATCC | CRL-2947 |
Syngeneic, Leukemia, L1210 | 578650 | ATCC | CRL-219 |
Syngeneic, Lymphoma, A20 | 578900 | ATCC | TIB-208 |
Syngeneic, Lung, KLN 205 | 578680 | ATCC | CRL-1453 |
Syngeneic, Lung, LL/2 | 578700 | ATCC | CRL-1642 |
Syngeneic, Melanoma, B16-F0 | 578800 | ATCC | CRL-6322 |
Syngeneic, Melanoma, B16-F10 | 578810 | ATCC | CRL-6475 |
Xenograft
Models
Human cancer cell line이나 (CDX Model) 환자 유래 암 조직 (PDX Model)을 피하 이식 (Subcutaneous) 또는
조직 이식 (Orthotopic)을 통해 면역 결핍 생쥐에 주입하며,
항암제 효능 및 독성을 평가합니다. 약물 효능평가를 위한 가장 대표적인 동물 모델로서, SOC drug에 대한 Reference data를 보유 및 제공합니다. Orthotopic mouse model의 경우, 세포를 암 유래
조직에 직접 주입하여암 세포의 전이 과정을 관찰할 수 있습니다.
Xenograft Models | |||
Model Name | Item no. | Cell Lines used in mouse model | |
Xenograft, Askin’s tumor, SK-N-MC | 581100 | ATCC | HTB-10 |
Xenograft, Bladder, UM-UC-3 | 580500 | ATCC | CRL-1749 |
Xenograft, Brain, U87-MG | 579500 | ATCC | HTB-14 |
Xenograft, Breast, BT-474 | 579700 | ATCC | HTB-20 |
Xenograft, Breast, HCC-1428 | 579800 | ATCC | CRL-2327 |
Xenograft, Breast, JIMT-1 | 579900 | AddexBio | C0006005 |
Xenograft, Breast, MCF7 | 580000 | ATCC | HTB-22 |
Xenograft, Breast, MDA-MB-231 | 580020 | ATCC | HTB-26 |
Xenograft, Breast, T-47D | 580030 | ATCC | HTB-133 |
Xenograft, Bone, U2OS (Coming soon) | |||
Xenograft, Colon, HCT 116 | 580090 | ATCC | CCL-247 |
Xenograft, Colon, HT-29 | 580100 | ATCC | HTB-38 |
Xenograft, Colon, SW480 | 580150 | ATCC | CCL-228 |
Xenograft, Gastric, NCI-N87 | 582400 | ATCC | CRL-5822 |
Xenograft, Head and Neck, SAS | 580300 | JCRB | JCRB0260 |
Xenograft, Kidney, A-498 | 580400 | ATCC | HTB-44 |
Xenograft, Kidney, ACHN | 580450 | ATCC | CRL-1611 |
Xenograft, Leukemia, HL-60 | 580600 | ATCC | CCL-240 |
Xenograft, Leukemia, MV-4-11 | 580650 | ATCC | CRL-9591 |
Xenograft, Leukemia, SKM-1 | 580670 | JCRB | JCRB0118 |
Xenograft, Leukemia, THP-1 | 580680 | ATCC | TIB-202 |
Xenograft, Liver, Hep3B (Hep 3B2.1-7) | 580700 | ATCC | HB-8064 |
Xenograft, Liver, PLC5 (PLC/PRF/5) | 580730 | ATCC | CRL-8024 |
Xenograft, Lung, A549 | 580900 | ATCC | CCL-185 |
Xenograft, Lung, NCI-H1975 | 580910 | ATCC | CRL-5908 |
Xenograft, Lung, NCI-H209 | 580940 | ATCC | HTB-172 |
Xenograft, Lung, NCI-H460 | 580950 | ATCC | HTB-177 |
Xenograft, Lung, NCI-H526 | 580960 | ATCC | CRL-5811 |
Xenograft, Lung, PC-9 | 580970 | Sigma Aldrich | 90071810 |
Xenograft, Lymphoma, U-937 | 581000 | ATCC | CRL-1593.2 |
Xenograft, Lymphoma, SU-DHL-5 | 581010 | ATCC | CRL-2958 |
Xenograft, Melanoma, A-375 | 582100 | ATCC | CRL-1619 |
Xenograft, Melanoma, SK-MEL-5 | 582110 | ATCC | HTB-70 |
Xenograft, Myeloma, RPMI 8226 | 582200 | ATCC | CCL-155 |
Xenograft, Neuroblastoma, IMR-32 | 582300 | ATCC | CCL-127 |
Xenograft, Neuroblastoma, SK-N-AS | 582310 | ATCC | CRL-2137 |
Xenograft, Ovary, SK-OV-3 | 581300 | ATCC | HTB-77 |
Xenograft, Ovary, OVCAR-3 | 581330 | ATCC | HTB-161 |
Xenograft, Pancreas, Bx-PC3 | 581520 | ATCC | CRL-1687 |
Xenograft, Pancreas, MIA PaCa-2 | 581500 | ATCC | CRL-1420 |
Xenograft, Pancreas, PANC-1 | 581540 | ATCC | CRL-1469 |
Xenograft, Prostate, 22Rv1 | 581920 | ATCC | CRL-2505 |
Xenograft, Prostate, DU-145 | 581930 | ATCC | HTB-81 |
Xenograft, Prostate, LNCaP clone FGC | 581950 | ATCC | CRL-1740 |
Xenograft, Prostate, PC-3 | 581900 | ATCC | CRL-1740 |
Xenograft, Skin, A-431 | 582000 | ATCC | CRL-1555 |
Orthotopic Models | |||
Model Name | Item no. | Cell Lines used in mouse model | |
Syngeneic, Breast, Orthotopic, Metastasis, 4T1 | 578591 | ATCC | CRL-2539 |
Xenograft, Brain, Orthotopic, U87-MG-Luc | 579502 | PerkinElmer | BW124577 |
Xenograft, Breast, Orthotopic, MDA-MB-231 | 580021 | ATCC | HTB-26 |
Xenograft, Breast, Orthotopic, MDA-MB-231-Luc | 580022 | JCRB | JCRB1559 |
Xenograft, Orthotopic, Leukemia, HL-60 | 580601 | ATCC | CCL-240 |
Xenograft, Orthotopic, Leukemia, MV-4-11 | 580651 | ATCC | CRL-9591 |
Xenograft, Orthotopic, Lymphoma, U-937 | 581001 | ATCC | CRL-1593.2 |
Xenograft, Pancreas, Orthotopic BxPC-3 | 581521 | ATCC | CRL-1687 |
Xenograft, Pancreas, Orthotopic, Bx-PC3-Luc | 581522 | PerkinElmer | BW125058 |
Xenograft, Pancreas, Orthotopic MIA PaCa-2 | 581501 | ATCC | CRL-1420 |
Xenograft, Pancreas, Orthotopic PANC-1 | 581541 | ATCC | CRL-1469 |
PDX Models | |||
Model Name | Item no. | Specimen information | |
Xenograft, Colon, PDX T18 | 585100 | KRAS mutation | Stage IV A |
Xenograft, Pancreas, PDX, (coming soon) | |||
Xenograft, Gastric, PDX, (coming soon) | |||
데이터 자료를 살펴보세요
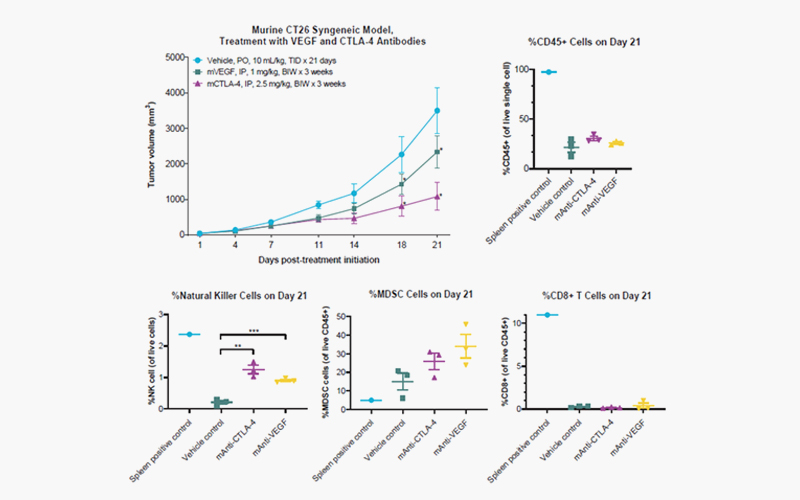
[Syngeneic
colorectal CT26 model (#578600), tumor growth over time (Day). Murine
colorectal CT26 cells, at 5 x 105 cells/mouse, were subcutaneously
implanted into female BALB/c mice. Treatment was initiated on Day 1 upon
randomization when the mean tumor volume reached 40-80 mm3. Murine
anti-VEGF and CTLA-4 antibodies were intraperitoneally administered twice per
week (top left panel), followed by TILs analysis on Day 21.The percentage of
live and murine CD45 positive cell populations are plotted (top right
panel).The percentage of murine natural killer (NK) cells, myeloid-derived
suppressor cells (MDSC), and CD8 positive T-cells are plotted with respect to
live CD45 positive cells for each group.(*): Indicates a significant (p <
0.05) reduction in the mean tumor volume compared to vehicle control as
determined by two-way ANOVA followed by Bonferroni correction. Significant
difference (**p < 0.01 and **p < 0.001) in the population of immune cells
compared to vehicle control as determined by one-way ANOVA followed by
Dunnett’s test. Error bars are SEM values.
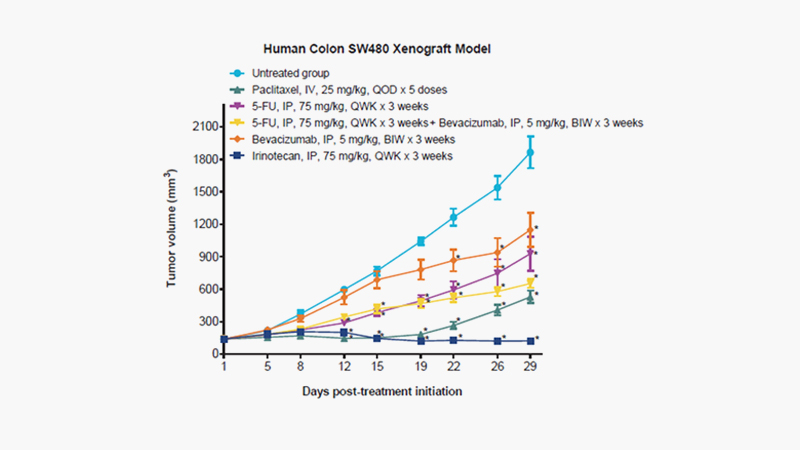
[Xenograft
model of human Duke’s type B colorectal carcinoma SW480 (#580150), tumor growth
over time (Day). Female nu/nu mice, 6 weeks of age, were subcutaneously
(SC) implanted with viable human colorectal carcinoma SW480 cells at 5 x 106
cells/mouse (0.2 mL/animal with 50% Matrigel). Treatment was initiated on Day 1
upon randomization when the mean tumor volume reached 100-150 mm3. Paclitaxel
at 25 mg/kg was intravenously (IV) administered once every other day for a
total of 5 doses (QOD x 5).Fluorouracil (5-FU) at 75 mg/kg and Bevacizumab at 5
mg/kg were each intraperitoneally (IP) administered with respect to once (QWK)
and twice (BIW) per week for 3 consecutive weeks. A combination therapy of 5-FU
and Bevacizumab was assessed to compare the effect with monotherapies. Irinotecan
at 75 mg/kg IP administered QWK x 3 weeks. Tumor growth, tumor volume by length
x (width)2 x 0.5, was measured thrice per week from Day 1 (treatment
initiation) till the study end date. Error bars are SEM values.(*): Indicates a
significant (p < 0.05) reduction in mean tumor volume compared to vehicle
control as determined by two-way ANOVA followed by Bonferroni correction. Error
bars are SEM values.]
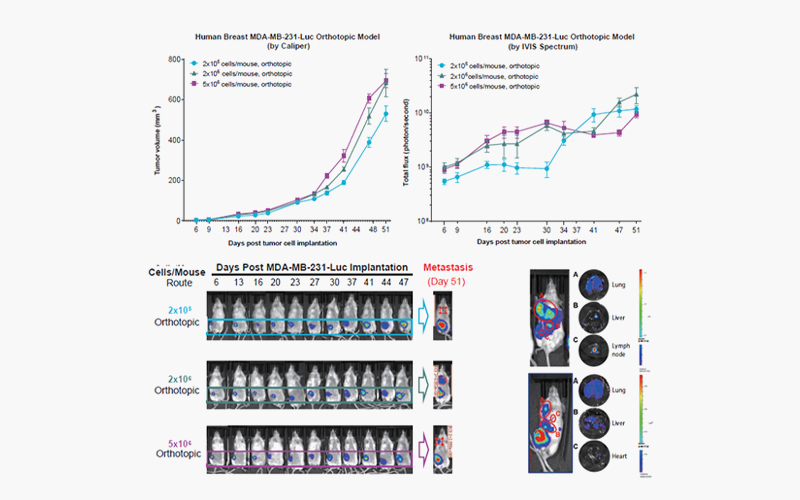
[Orthotopic
model of human triple-negative breast carcinoma MDA-MB-231. Female NPG
mice, 6 weeks of age, were orthotopically implanted with viable human TNBC
MDA-MB-231 cells at the selected target cell density into the mammary fat pad
at 0.05 mL/animal. Tumor growth was measured by IVIS and caliper twice per week
throughout the study progression. Both the caliper and IVIS versions with MDA-MB-231-Luc
(luciferase labeled) cells are available (#580022). Representative luciferase
signal in the bottom left panel throughout the course of the study. Multiple
organ metastases are found, as shown in the bottom right panel. The xenograft
(#580020) and orthotopic (#580021) models with the parental MDA-MB-231
(unlabeled cells) are also available for selection.]
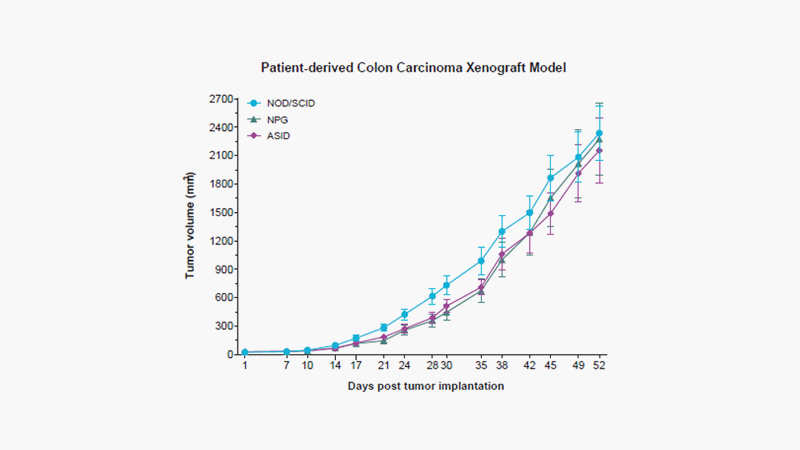
[Patient-derived xenograft model with colorectal carcinoma, tumor growth over time (Day). Female ASID, NPG, and NOD/SCID mice, 8 weeks of age, were subcutaneously (SC) implanted with tumor fragments (passage number 3) obtained from an age 45 male patient with adenocarcinoma colorectal cancer at the upper rectum, stage IV A, poorly differentiated. Tumor growth, tumor volume by length x (width)2 x 0.5, was measured twice per week during the study progression.]
서비스는 이런 절차로 진행됩니다
-
유선 및 방문상담

서비스상담
실험항목 및 조건 논의 -
최초 견적서 전달

계약
실험의뢰서(CSF) 작성
(필요시) 계약서 작성 -
실험 물질 전달

시료 준비
(택배 혹은 방문수령) -
매주 FedEx 발송

시료 발송
Tracking no. 안내
Report due date 안내 -
실험종료 안내

결과제공
최종 보고서 전달
결과 검토 및 상담
결제 진행

![[Brochure] In Vivo Oncology Models for Drug Discovery. In Vivo and Ex Vivo Services to Expedite Preclinical Testing [Brochure] In Vivo Oncology Models for Drug Discovery. In Vivo and Ex Vivo Services to Expedite Preclinical Testing](/upload/reference/cd98f00b2093115.jpg)
![[Poster] Targeting Squalene Epoxidase for Breast Cancer Brain Metastasis: The Evaluation of a New Therapeutic Target for Brain Extravasation and Colonization Using Animal Models [Poster] Targeting Squalene Epoxidase for Breast Cancer Brain Metastasis: The Evaluation of a New Therapeutic Target for Brain Extravasation and Colonization Using Animal Models](/upload/reference/cd98f00b2029678.png)